Toxic heavy metals are all around us, in bedrock and in the...
Read MoreContaminants
in Baby Foods
Updated on April 24, 2025: News Contaminants
Infant and Young Child Feeding and Chemical Residues
Parents, carers and health professionals are rightly concerned about all the problems in infant and young child feeding in a contaminated world. The repeated failures in systems to protect the safety of children’s food and the presence of toxic substances and chemical residues in food can all have harmful effects on children’s health.
In this context, parents, carers and health professionals need objective and independent information on the risks and dangers of environmental pollution.
Key points:
- Every human body is estimated to contain up to 200 man-made chemicals. All human beings and wildlife carry this ‘body burden’ of industrial chemicals. They are persistent and accumulate in organisms as they move higher up the food chain.
- Both men and women carry this body burden from exposure to chemical substances. Many of these are fat soluble, meaning they dissolve in body fat, and so their levels can be measured in body tissues and fluids: blood, serum, urine, sperm, human umbilical cord blood and breastmilk.
- Research has shown that these chemicals can have a harmful impact in humans and wildlife. Some of them are known to cause cancer, some are neurotoxic, some impair the immune and endocrine system, are associated with the development of chronic diseases and may even have inter-generational effects on reproduction. Little is known about how they interact in combination with each other.
- Infants and young children are particularly vulnerable to the effects of exposure to chemicals because they are at the most sensitive stages of human development. Prenatal exposure to chemicals, when a baby is in its mother’s womb, is of greater concern than postnatal exposure, when a baby is exposed after birth to residues of chemicals found in breastmilk or to chemicals in formula and feeding bottles.
- Breastmilk contains protective agents and helps the child develop a strong immune system. Breastfeeding can mitigate the effects of chemical exposure in the womb, whereas formula feeding does not afford any protection or mitigation.
- Formula feeding leaves a heavy carbon footprint and contributes significantly to contamination of the environment with these chemicals. Policy-makers should be alerted to the need to legislate to reduce the waste and contamination caused by the production, distribution and disposal of non-biodegradable formula feeding products.
- Infant, follow-up and toddler formula, as well as all sorts of industrial baby foods, let alone industrial foods for older children and adults, can be contaminated, often at higher levels, by the same chemical residues found in breastmilk. Exposure to some of these substances can occur also through the polycarbonate plastic bottles and cups used to give these foods and drinks to infants and young children, or by their transfer to foods and beverages from feeding bottles, and the lining of tin cans and packages.
- Powdered infant formula can be intrinsically contaminated with bacteria. Reconstituted powdered infant formula can be contaminated by the same and different bacteria through incorrect preparation, handling and storage. To avoid damage caused by these sorts of contamination, it is important to follow thoroughly the WHO recommendations for the preparation of powdered infant formula.1
- Except in cases of industrial disasters and of exceedingly high levels of dangerous residues after industrial disasters, IBFAN emphasises the recommendation to protect, promote and support breastfeeding. This holds true even when there is evidence of the presence of chemical residues in breastmilk because the benefits of breastfeeding far outweigh any possible harm. Furthermore, IBFAN recommends that the debate about the detection of chemical residues in breastmilk should not unduly influence a mother’s decision to breastfeed.
- Future parents and carers of children should be informed of practical ways to reduce exposure to toxic chemicals. All of us should campaign to reduce the amount of chemicals in our environment and to counter the powerful lobby of the plastics and chemical industries.
Key IBFAN messages
- Breastfeeding is the norm for feeding infants and young children; any other feeding mode is inferior. Early (within one hour from birth), exclusive (for six months) and then continued (with adequate and safe complementary foods) breastfeeding for two years and beyond, provides optimal, unique and perfectly balanced nutrition for a baby even in a contaminated environment.
- Breastfeeding affords many irreplaceable positive health effects for both mother and child, economic advantages for families, communities, societies and health systems, and ecological advantages for the environment.
- Pregnant women and breastfeeding mothers have the right to receive full and unbiased information. They should thus be alerted to the problems caused by chemical contaminants in their body fluids, and should fight, in alliance with breastfeeding support and environmental groups, for the reduction of chemical residues in food and the environment.
- All citizens should work to raise awareness of the dangers of environmental pollution, including that brought about by formula and bottle feeding and by the undue use of industrial baby foods, and should demand their governments to act in their best interest.
IBFAN Statement: Infant and Young Child Feeding and Chemical Residues
Authors: The lead author for this IBFAN statement is Dr. Adriano Cattaneo, with input provided by members of IBFAN's working group on chemical and microbiological contamination of infant feeding products.
2013
This material is part of a document published by IBFAN in 2013, which contains information based on up-to-date evidence from peer-reviewed scientific research. To read the full document, click the button.
Urgent Matters
PFAS - The problem
PFAS (polyfluoroalkyl substances) is a group of thousands of “forever chemicals”, so called because they are not bio-degraded and persist therefore in the environment. The first PFAS were used by the industry and were released in the environment in the mid ‘50s of last century; they are still with us. Their number can be increased almost indefinitely because the original molecule can be easily manipulated to obtain new ones, with longer or shorter chemical chains. If some PFAS, such as PFOS and PFOA, are banned because they are shown to cause cancer or other harms to health and the planet (and it can take ages before the evidence is put together), the chemical industry will easily replace them with new ones. All the other industries (plastic, garment, furniture, transport, food packaging, cookware, cosmetics, electronics, etc.) need PFAS for their products, to make them resistant to water, stains and heat, so that demand for the chemical industry is constant.
PFAS act as endocrine disruptors. By interfering with hormones, they cause harm to thyroid, liver, and immune system. Among others, they interfere with hormones related with our reproductive systems. Exposure to PFAS may have lifelong impact because they bio-accumulate in our bodies and act during pregnancy, early childhood, and adolescence when cells divide and multiply rapidly. This may be the reason why some PFAS (PFOS and PFOA, for example) have already been shown to be associated with cancers of reproductive and related organs, such as the breast.

When an association with cancer or cardiovascular disease is ascertained, one or more PFAS may be banned by national and supranational authorities (the EU, for example). Unfortunately, banned PFAS are quickly replaced by similar and untested molecules.
PFAS are present in the food chain. They are lipophiles; therefore, their concentration is higher in fat-rich food, such as fish, meat, eggs, milk, and milk products. When they reach human beings, who are at the top of the food chain, their levels are higher in fat-rich tissues, such as the breast, than in blood and urine, for example. PFAS can be detected in breastmilk, but also in cow milk and cow milk-based formula. Their concentration is proportional to the concentration of PFAS in the diet; carnivorous animals and human beings ingest more PFAS than vegetarian ones. It is also proportional to the concentration of PFAS in drinking water. For example, in the female population of an area in Northeastern Italy where water has been heavily polluted by a PFAS industry for years, mothers have on average a high concentration of PFAS in breastmilk, but those drinking water from polluted wells and with a mostly animal diet have much higher than average concentrations.



PFAS and infant feeding
As for any other chemical contaminant in breastmilk, several points must be considered before recommending replacement of breastmilk with formula.
First, the infant born to a mother with PFAS in breastmilk has already been exposed to the same substance in utero. It is well known that chemical substances cause more harm when cells divide rapidly, i.e. early more than late in pregnancy, in pregnancy more than in the newborn period, in the newborn period more than later in infancy, and so on. This means that if PFAS cause harm, that harm has already been caused before birth. Any harm caused will depend on the type of PFAS (some are more harmful than others) and its concentration. If concentrations are low, breastfeeding is in any case recommended.
Second, it is well known that breastmilk and breastfeeding are beneficial to health, nutrition, growth, and development. In most cases, these benefits will be higher than any harm caused by PFAS. Once again, it will depend on the type of PFAS and its concentration in breastmilk. At very high concentrations, such as those found in a minority of mothers in the already mentioned area of Northeastern Italy, it may be advisable to avoid or interrupt breastfeeding and turn to formula feeding. In all the other cases breastfeeding continues to be the right choice, especially for its positive effects on the immune system.
Third, most formulae are cow milk-based. Before switching to formula feeding, it would be wise to ensure that formula is PFAS-free. Also, powder formula needs to be reconstituted with water, and if drinking water contains PFAS we are back to square zero. The alternative may be to use bottled water; but unfortunately, this may also contain PFAS. Note that well-known manufacturers of formula, who are engaged in the production of bottled water, are accused of practices to keep hiding the low quality of their water sources for years. In addition, at around six months of age infants will be introduced to complementary foods and drinking water. If these foods and waters are the same that led mothers to have PFAS in their blood, womb and breastmilk, avoiding breastfeeding in the first six months of life will have little effect.

The solution
As already mentioned, when a PFAS is shown to cause harm to people, it may be banned. Unfortunately, bans of single PFAS are only partly effective, because the banned molecules are quickly replaced by new ones and it will take years before the harmful effect of the new molecules are detected. The final solution would be a total ban of all PFAS (Figure 3), as advocated by a network of 138 civil society organizations in Europe. However, this would mean a total change in our way of life: the way we prepare food, the cookware we use, the furniture and garments we buy, etc. In summary, most industrial processes would need to be changed if it becomes impossible to use PFAS.
A total ban of PFAS may also be ineffective if the chemical industry devised non-PFAS chemicals to replace PFAS. There is no guarantee that the new non-PFAS chemicals with PFAS properties would be safe. History tells us that the harms of any chemical become visible only ages after the new product is introduced in our environment.
Meanwhile, corporate lobbies “are targeting the European Commission to protect their PFAS substances, products, equipment, and profits, despite the overwhelming evidence of the disastrous human health and environmental consequences of the pollution that they cause.”[1] To protect all human and non-human bodies, and the environment in which they live, we should not allow these corporate interests to prevail.
High PFAS Contamination:
Greenpeace Italy Report Reveals Widespread Presence in Drinking Water
A report recently published by Greenpeace Italy shows that 79% of 260 samples of potable drinking water obtained between September and October 2024 in 235 towns spread all over the country (Figure 1) contain PFAS. The findings refer to 58 PFAS substances, many more than the 20 ones for which monitoring is compulsory according to the European Union (EU). A similar situation can be assumed worldwide in countries and territories with high population and industry density (Figure 2). Data comes from 45,000 samples of surface and ground water; in 31% of them PFAS were detected, the average obviously hiding higher rates of contaminations in many places. In 11 EU countries, PFAS were detected in most drinking waters, including some bottled mineral waters.
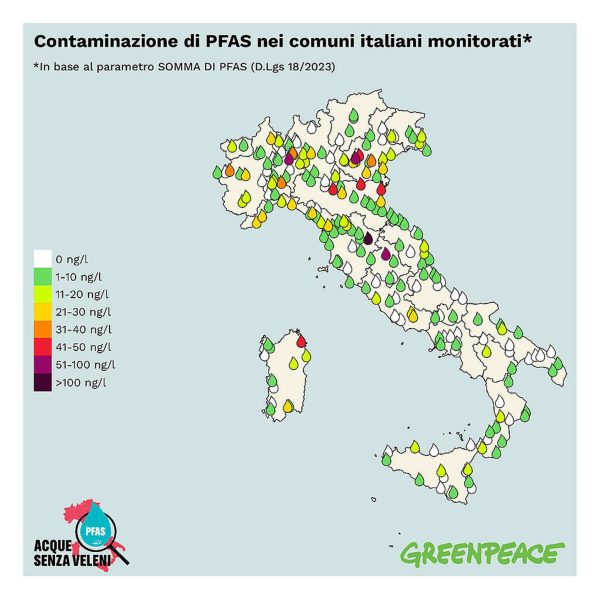
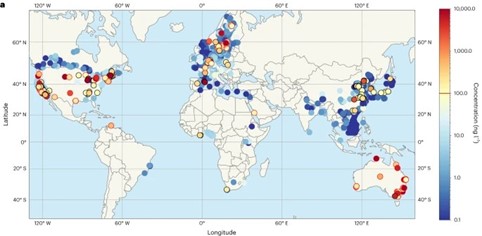
It is not difficult to conclude that PFAS are everywhere, and forever. If there are PFAS in all water sources, there will be PFAS in soils, plants, vegetables, insects, animals, and human beings. If water is contaminated, all bodies are contaminated.
Check out the statement from IBFAN during the 156th session of the WHO Executive Board (EB156)
Geneva, Switzerland | 3–11 February, 2025
The impact of chemicals, waste and pollution on human health
Anna Koronkiewicz-Wiórek (IBFAN Poland) – 08/02/2025
IBFAN appreciates WHO’s support for this work. Prenatal and postnatal exposure to toxic chemicals can harm children’s health. In some highly contaminated areas even breastfeeding may be at risk. However, in most cases the benefits of breastfeeding will be greater than harm caused by such contamination.
Breastfeeding confers immuno-protective factors and helps build children’s lifelong immune system. Infant feeding products and packaging are inherently risky and contain plastics and Endocrine Disrupting Chemicals which impact ON babies’ immune, reproductive and ENDOCRINE systems.
WHO must continue work on the Global Plastics Treaty to develop a legally binding instrument on plastic pollution and protect the health of all children and their environment from toxic chemicals, while ensuring parents receive sound independent information free of commercial influence.
News about Contaminants in Baby Foods
GLOBAL PLASTICS TREATY: BABIES, YOUNG CHILDREN AND WOMEN ARE MOST AT RISK
Babies, young children and their mothers are most at risk from toxic...
Read MoreCOP 29 – THREATS TO THE WORLD’S FOOD SUPPLY – THE ROLE OF BREASTFEEDING
The threats to the world’s food supply are numerous and often interconnected,...
Read More



